Work hardening is used extensively to produce strain-hardened tempers of the non-heat-treatable alloys. The severely cold worked or full-hard condition (H18 temper) is usually obtained with cold work equal to about 75% reduction in area. The H19 temper identifies products with substantially higher strengths and greater reductions in area. The H16, H14, and H12 tempers are obtained with lesser amounts of cold working, and they represent three-quarter-hard, half-hard, and quarter-hard conditions, respectively.
A combination of strain hardening and partial annealing is used to produce the H28, H26, H24, and H22 series of tempers; the products are strain hardened more than is required to achieve the desired properties and then are reduced in strength by partial annealing.
A series of strain-hardened and stabilized tempers – H38, H36, H34, and H32 – are employed for aluminum-magnesium alloys. In the strain-hardened condition, these alloys tend to age soften at room temperature. Therefore, they are usually heated at a low temperature to complete the age-softening process and to provide stable mechanical properties and improved working characteristics.
Products hardened by cold working can be restored to the O temper, a soft, ductile condition, by annealing. Annealing eliminates strain hardening, as well as the changes in structure that are the result of cold working.
The distorted, dislocated structure resulting from cold working of aluminum is less stable than the strain-free, annealed state, to which it tends to revert. In zone-refined aluminum, this reversion may take place at room temperature. Lower-purity aluminum and commercial aluminum alloys undergo these structural changes only with annealing at elevated temperatures. Accompanying the structural reversion are changes in the various properties affected by cold working. These changes occur in several stages, according to temperature or time, and have led to the concept of different annealing mechanisms or processes. The first of these, occurring at the lowest temperatures and shortest times of annealing, is known as the recovery process.
Recovery
Structural changes occurring during the recovery of polygonization and subgrain formation has been obtained by x-ray diffraction and confirmed with the electron microscope. The electron micrographs may show the change in structure that accompanies advanced recovery. The reduction in the number of dislocations is greatest at the center of the grain fragments, producing a subgrain structure with networks or groups of dislocations at the subgrain boundaries. With increasing time and temperature of heating, polygonization becomes more nearly perfect and the subgrain size gradually increases. In this stage, many of the subgrains appear to have boundaries that are free of dislocation tangles and concentrations.
The decrease in dislocation density caused by recovery-type annealing produces a decrease in strength and other property changes. The effects on the tensile properties of 1100 alloy are shown in Fig. 1. At temperatures through 450°F (230°C), softening is by a recovery mechanism. It is characterized by an initial rapid decrease in strength and a slow, asymptotic approach to a strength that is lower, the higher the temperature.
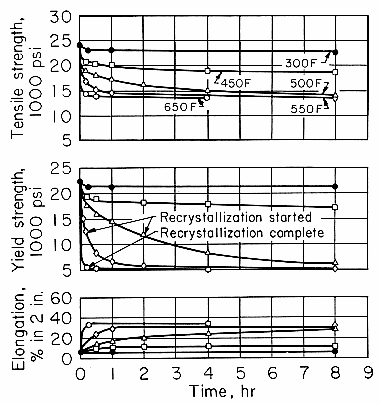
Fig. 1. Isothermal annealing curves for 1100-H18 sheet.
Recrystallization
Recrystallization is characterized by the gradual formation and appearance of a microscopically resolvable grain structure. The new structure is largely strain-free. There are few if any dislocations within the grains and no concentrations at the grain boundaries. Recrystallization occurs with longer times or higher heating temperatures than do the recovery effects described in the preceding section, although some overlapping of the two processes is usual.
Recrystallization depends upon time and temperature. This relationship can be expressed by a rate equation of the type:
1/t = ke-a/T
where t is time, T is the absolute temperature, e is the base of natural logarithms, and k and a are constants.
The constant a is frequently replaced by Q/R, where R is the gas constant and Q is an energy term, similar to an activation energy. Aluminum alloys generally show good agreement with this time-temperature relationship except when secondary reactions interfere, such as the solution or precipitation of intermetallic phases at annealing temperatures.
Composition also influences the recrystallization process. This is particularly true when various elements are added to extreme purity aluminum; almost any added impurity or alloying element will raise the recrystallization temperature substantially. For commercial-purity aluminum and commercial alloys, however, normal variations in composition have little effect on recrystallization behavior. Extensively cold worked commercial alloys usually can be recrystallized by heating for several hours at 650 to 775°F (340 to 410°C).
Grain size is also strongly affected by composition. Generally, common alloying elements and impurities such as Cu, Fe, Mg, and Mn decrease grain size. The effects of elements of limited solubility, such as Cr, Fe, and Mn, are influenced by the compounds they form with each other and with other elements, and by their distribution in the structure.
The recovery process is not accompanied by any significant change in preferred orientation or texture of the deformed metal. However, the new grains formed by recrystallization frequently develop in orientations that differ from the principal components of the deformation texture. This re-orientation has been extensively studied in rolled sheet and varies considerably with the past history and the composition of the alloy.
Recrystallization produces further changes in the properties of the deformed and recovered metal. These continue until annealing and recrystallization are complete. The properties then are those of the original, unstrained metal, except as they are changed by differences in grain size and preferred orientation. In heat treatable alloys, annealing also may be accompanied by precipitation and changes in solute concentration.
Recrystallization is also accompanied by a further decrease in stored energy, as measured calorimetrically, as well as by complete elimination of residual stresses.
Grain Growth After Recrystallization
Heating after recrystallization may produce grain coarsening. This can take one of several forms. The grain size may increase by a gradual and uniform coarsening of the microstructure. This is usually identified as “normal” grain growth.
It proceeds by the gradual elimination of small grains with unfavorable shapes or orientations relative to their immediate neighbors. This occurs readily in high-purity aluminum and its solid solution alloys, and can lead to relatively large, average grain sizes. Such grain growth is promoted by small recrystallized grains, high temperatures, and extensive heating. Some grain coarsening of this type also occurs in commercial aluminum alloys, but it is greatly restricted by finely divided impurity phases and by intermetallic compounds of elements, such as manganese and chromium that slows down the process pin the grain boundaries, and prevent further movement. Generally, these grains grow only at very high temperatures and may attain diameters of several inches.
Apparently, the normal growth-inhibiting effects of elements such as iron, manganese, and chromium are lost or modified at high temperatures, through solution or through changes in particle size and shape. Because of the high temperatures, the few grains that first lose or overcome these restraints grow rapidly and consume other potential growth centers, and in this manner, a few grains of very large size are formed.
In most alloys, high temperatures alone are not the only cause for “giant grains”: A small primary grain size and well-developed annealing texture are other factors that promote this form of grain growth.